Guided hierarchical co-assembly of soft patchy nanoparticles. Animal and plant cells are prominent examples of how nature constructs ever-larger units in a targeted, preprogrammed manner using molecules as building blocks. In nanotechnology, scientists mimic this ‘bottom-up’ technique by using the ability of suitably structured nano materials to ‘self-assemble’ into higher order architectures. Applying this concept, polymer scientists from Bayreuth, Aachen, Jena, Mainz, and Helsinki have recently published an article in the prestigious journal Nature that describes a new principle for the self-assembly of patterned nanoparticles. This principle may have important implications for the fundamental understanding of such processes as well as future technologies.
The research team is headed by Professor Axel Müller, who was holder of the Chair of Macromolecular Chemistry II at the University of Bayreuth until his retirement in 2012; he is now a Fellow of the Gutenberg Research College at Mainz University. The other members of the team are Dr. André Gröschel (previously at the University of Bayreuth, now Aalto University Helsinki), Tina Löbling and Dr. Holger Schmalz (University of Bayreuth), Dr. Andreas Walther (Interactive Materials Research Center at Aachen University), and Junior Professor Dr. Felix Schacher (Friedrich Schiller University Jena). The research was conducted at the University of Bayreuth and funded by the German Research Foundation (DFG) within the Collaborative Research Center 840 "From Particulate Nano-Systems to Mesotechnology."
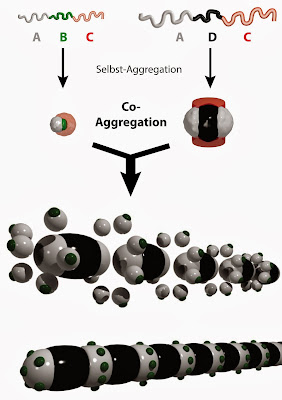
/©: Müller Research Group The self-assembly process described in Nature commences with chain-like macromolecules, so-called triblock terpolymers composed of three linear sections connected to form a chain-like structure A-B-C or A-D-C. The block in the middle has been marked green or black, respectively. Block A (gray) has to interact with other particles; block C (rose) is a corona controlling solubility. By way of self-aggregation the macromolecules formed nanoparticles, which by way of co-aggregation formed the next higher level in the hierarchy. This way a co-assembled superstructure develops, for which Müller's research team has coined the term "caterpillar micelles."
The self-assembly process commences with chain-like macromolecules with a size in the range of 10 to 20 nanometers. In chemistry, such macromolecules are called triblock terpolymers. They are composed of three linear sections (blocks) connected to each other in sequence. They are generated using a special synthetic process, i.e., the so-called "living polymerization," and are readily available to researchers. The research team was able to guide the triblock macromolecules into soft nanoparticles with a diameter of roughly 50 nanometers. The choice of solvents played a key role in this macromolecular self-assembly process. The solvents were precisely selected and used so that the varying solubility of the three blocks and the incompatibility of the polymers with one another contributed significantly to the quality of the desired interior structure of the nanoparticles.
The scientists applied this technique to two types of triblock terpolymers. These differed with regard to the chemical properties of the middle blocks. The block sequences of the macromolecules were A-B-C and A-D-C, respectively. The first results in nanoparticles with a single bonding site and tends to form spherical clusters, while the latter creates nanoparticles with two bonding sites and thus tends to form linear superstructures. Importantly, in both cases the structure of the nanoparticles is preprogrammed by the chemical structure of the source macromolecule in the same way as the structure of a protein is determined by its amino acid sequence.
However, the process of self-assembly does not end with the nanoparticles. If the nanoparticles formed by each type of macromolecule were left to their own, spherical superstructures would result on the one hand and linear superstructures on the other. Müller's team has developed and implemented a different approach. The nanoparticles with one and two bonding sites are mixed so that they aggregate together into a completely new superstructure in a process of co-assembly. In the final superstructure, the nanoparticles originating from the A-B-C molecules and nanoparticles formed by the A-D-C molecules alternate in a precisely defined pattern.
When viewed under a transmission electron microscope, the new superstructure bears a strong resemblance to a caterpillar larva, because it also consists of a series of clearly separate, regularly ordered sections. Müller's research team has thus coined the term "caterpillar micelles" for such co-assembled superstructures.
The research findings recently published in Nature represent a breakthrough in the field of hierarchical structuring and nano-engineering as it allows creating new materials by self-assemble preprogrammed particles. This could be a game changer, because so far only top-down procedures, i.e., extracting a microstructure from a larger complex, are widely accepted structuring processes. "The limitations of this technique will become all too apparent in the near future," explained Müller. "Only rarely is it possible to generate complex structures in the nanometer range."
However, a bottom-up principle of self-assembly based on that employed in nature could well represent the best way forward. One factor that makes this particularly attractive is the large number of macromolecules, which are readily available as building blocks. They can be used to incorporate specific properties in the resultant superstructures, such as sensitivity to environmental stimuli (e.g. temperature, light, electric and magnetic fields, etc.) or give them the ability to be switched on and off at will. Possible applications include nanolithography and the delivery of drugs in which the time and site of release of active substances can be preprogrammed. Here, the similarity to the structural principles of animal and plant cells becomes apparent again, where various properties are compartmentalized into areas of limited space.
The macromolecules carrying diverse functional segments can be hundreds of times smaller than a micrometer. The superstructures that such macromolecules produce have correspondingly high resolution. "Future technologies – such as tailor-made artificial cells, transistors, or components for micro/nano-robotics – may benefit significantly from this particularly delicate structuring," explained Müller. "The research findings we published in Nature do not yet have any immediate real-world applications. Nevertheless, the better we understand bottom-up processes starting with molecules in the nanometer range and moving on to the higher hierarchical levels in the micrometer range, the more likely future technologies will be within our grasp." The caterpillar micelles are in no way the only superstructures that can be produced with the self-assembling nanoparticles. "Such soft nanoparticles can be combined with inorganic or biological nano- and microparticles to create previously unknown materials with specific functions. The number of possible combinations is practically endless," concluded Müller.
Contact Professor Dr. Axel H. E. Müller Fellow of the Gutenberg Research Center (GRC) Institute of Organic Chemistry Johannes Gutenberg University D 55099 Mainz Tel +49 6131 39-22372












No comments:
Post a Comment