HOUSTON -- (Oct. 20, 2011) -- Giant flakes of graphene oxide in water aggregate like a stack of pancakes, but infinitely thinner, and in the process gain characteristics that materials scientists may find delicious.
A new paper by scientists at Rice University and the University of Colorado details how slices of graphene, the single-atom form of carbon, in a solution arrange themselves to form a nematic liquid crystal in which particles are free-floating but aligned.
That much was already known. The new twist is that if the flakes – in this case, graphene oxide – are big enough and concentrated enough, they retain their alignment as they form a gel. That gel is a handy precursor for manufacturing metamaterials or fibers with unique mechanical and electronic properties.
The team reported its discovery online this week in the Royal Society of Chemistry journal Soft Matter. Rice authors include Matteo Pasquali, a professor of chemical and biomolecular engineering and of chemistry; James Tour, the T.T. and W.F. Chao Chair in Chemistry as well as a professor of mechanical engineering and materials science and of computer science; postdoctoral research associate Dmitry Kosynkin; and graduate students Budhadipta Dan and Natnael Behabtu. Ivan Smalyukh, an assistant professor of physics at the University of Colorado at Boulder, led research for his group, in which Dan served as a visiting scientist.
"Graphene materials and fluid phases are a great research area," Pasquali said. "From the fundamental point of view, fluid phases comprising flakes are relatively unexplored, and certainly so when the flakes have important electronic properties.

Caption: A single flake of graphene oxide roughly 40 microns wide, seen under an electron microscope, sits atop a copper support. Such "giant" flakes form into a gel-like liquid crystal in solution.
Credit: Rice University/University of Colorado at Boulder. Usage Restrictions: None.
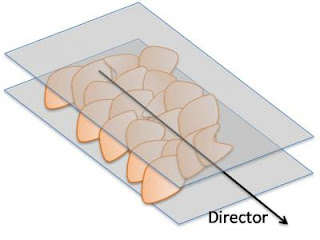
Caption: Graphene oxide flakes in a solution align themselves with a director, a dimensionless vector that represents the preferred orientation of particles in a liquid crystal.
Credit: Rice University/University of Colorado at Boulder. Usage Restrictions: None. | "From the application standpoint, graphene and graphene oxide can be important building blocks in such areas as flexible electronics and conductive and high-strength materials, and can serve as templates for ordering plasmonic structures," he said.
By "giant," the researchers referred to irregular flakes of graphene oxide up to 10,000 times as wide as they are high. (That's still impossibly small: on average, roughly 12 microns wide and less than a nanometer high.) Previous studies showed smaller bits of pristine graphene suspended in acid would form a liquid crystal and that graphene oxide would do likewise in other solutions, including water.
This time the team discovered that if the flakes are big enough and concentrated enough, the solution becomes semisolid. When they constrained the gel to a thin pipette and evaporated some of the water, the graphene oxide flakes got closer to each other and stacked up spontaneously, although imperfectly.
"The exciting part for me is the spontaneous ordering of graphene oxide into a liquid crystal, which nobody had observed before," said Behabtu, a member of Pasquali's lab. "It's still a liquid, but it's ordered. That's useful to make fibers, but it could also induce order on other particles like nanorods."
He said it would be a simple matter to heat the concentrated gel and extrude it into something like carbon fiber, with enhanced properties provided by "mix-ins."
Testing the possibilities, the researchers mixed gold microtriangles and glass microrods into the solution, and found both were effectively forced to line up with the pancaking flakes. Their inclusion also helped the team get visual confirmation of the flakes' orientation.
The process offers the possibility of the large-scale ordering and alignment of such plasmonic particles as gold, silver and palladium nanorods, important components in optoelectronic devices and metamaterials, they reported. |
Behabtu added that heating the gel "crosslinks the flakes, and that's good for mechanical strength. You can even heat graphene oxide enough to reduce it, stripping out the oxygen and turning it back into graphite."
###
Co-authors of the paper are Angel Martinez and Julian Evans, graduate students of Smalyukh at the University of Colorado at Boulder.
The Institute for Complex Adaptive Matter, the Colorado Renewable and Sustainable Energy Initiative, the National Science Foundation, the Air Force Research Lab, the Air Force Office of Scientific Research, the Welch Foundation, the U.S. Army Corps of Engineers Environmental Quality and Installation Program and M-I Swaco supported the research.
Contact: Mike Williams
mikewilliams@rice.edu 713-348-6728
Rice University












No comments:
Post a Comment