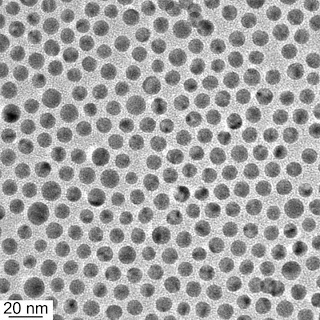
Scientists with the Lawrence Berkeley National Laboratory (Berkeley Lab) and the University of California (UC) Berkeley have directed the first self-assembly of nanoparticles into device-ready materials. Through a relatively easy and inexpensive technique based on blending nanoparticles with block co-polymer supramolecules, the researchers produced multiple-layers of thin films from highly ordered one-, two- and three-dimensional arrays of gold nanoparticles. Thin films such as these have potential applications for a wide range of fields, including computer memory storage, energy harvesting, energy storage, remote-sensing, catalysis, light management and the emerging new field of plasmonics.
“We’ve demonstrated a simple yet versatile supramolecular approach to control the 3-D spatial organization of nanoparticles with single particle precision over macroscopic distances in thin films,” says polymer scientist Ting Xu, who led this research. “While the thin gold films we made were wafer-sized, the technique can easily produce much larger films, and it can be used on nanoparticles of many other materials besides gold.”
Xu holds joint appointments with Berkeley Lab’s Materials Sciences Division and UC Berkeley’s Departments of Materials Sciences and Engineering, and Chemistry. She is the corresponding author of a paper describing this research in the journal Nano Letters titled “Nanoparticle Assemblies in Thin Films of Supramolecular Nanocomposites.” Co-authoring the paper were Joseph Kao, Peter Bai, Vivian Chuang, Zhang Jiang and Peter Ercius.
Nanoparticles can be thought of as artificial atoms with unique optical, electrical and mechanical properties. If nanoparticles can be coaxed into routinely assembling themselves into complex structures and hierarchical patterns, similar to what nature does with proteins, devices a thousand times smaller than those of today’s microtechnologies could be mass-produced.
In this latest study, Xu and her colleagues incorporated gold nanoparticles into solutions of block co-polymer supramolecules to form films that ranged in thickness between 100 to 200 nanometers. The nanocomposite films featured microdomains in one of two common morphologies – lamellar or cylindrical. For the lamellar microdomains, the nanoparticles formed hexagonally-packed 2-D sheets that were stacked into multiple layers parallel to the surface. For the cylindrical microdomains, the nanoparticles formed 1-D chains (single particle width) that were packed into distorted hexagonal lattices in parallel orientation with the surface.
“Upon incorporation of nanoparticles, the block co-polymer supramolecules experience conformational changes, resulting in entropy that determines the placement and distribution of the nanoparticles, as well as the overall morphology of the nanocomposite thin films,” Xu says. “Our results indicate that it should be possible to generate highly-ordered lattices of nanoparticles within block co-polymer microdomains and obtain 3-D hierarchical assemblies of nanoparticles with precise structural control.”
The inter-particle distance between gold nanoparticles in the 1-D chains and the 2-D sheets was 8 to 10 nanometers, which raises intriguing possibilities with regards to plasmonics, the phenomenon by which a beam of light is confined in ultra-cramped spaces. Plasmonic technology holds great promise for superfast computers and optical microscopy, among other applications. However, a major challenge for developing plasmonics has been the difficulty of fabricating metamaterials with noble metal nanoparticles such as gold.
“Our gold thin films display strong plasmonic coupling along the inter-particle spacing in the 1-D chains and 2-D sheets respectively,” Xu says. “We should therefore be able to use these films to investigate unique plasmonic properties for next-generation electronic and photonic devices. Our supramolecular technique might also be used to fabricate plasmonic metamaterials.”
This research was supported by the U.S. Department of Energy Office of Science.
# # #
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
Contact: Lynn Yarris lcyarris@lbl.gov 510-486-5375 DOE/Lawrence Berkeley National Laboratory