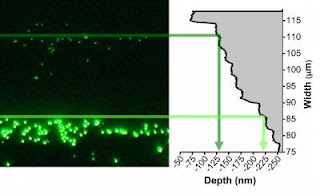
OAK RIDGE, Tenn., — Thin layers of oxide materials and their interfaces have been observed in atomic resolution during growth for the first time by researchers at the Center for Nanophase Materials Sciences at the Department of Energy's Oak Ridge National Laboratory, providing new insight into the complicated link between their structure and properties.
"Imagine you suddenly had the ability to see in color, or in 3-D," said the CNMS's Sergei Kalinin. "That is how close we have been able to look at these very small interfaces."
The paper was published online in ACS Nano with ORNL's Junsoo Shin as lead author.
A component of magnetoelectronics and spintronics, oxide interfaces have the potential to replace silicon-based microelectronic devices and improve the power and memory retention of other electronic technologies.
"Instead of seeing a perfectly flat, square lattice that scientists thought these interfaces were before, we found a different and very complicated atomic ordering," said Baddorf. "We really need to reassess what we know about these materials."
Oxides can be used in different combinations to produce unique results. For instance, isolated, two oxides may be insulators but together the interface may become conductive. By viewing the atomic structure of one oxide, scientists can more effectively couple oxides to perform optimally in advanced technological applications such as transistors.
Kalinin says the correct application of these interface-based materials may open new pathways for development of computer processors and energy storage and conversion devices, as well as understanding basic physics controlling these materials.
"In the last 10 years, there has been only limited progress in developing beyond-silicon information technologies," Kalinin said. "Silicon has limitations that have been reached, and this has motivated people to explore other options."
Atomic resolution of interface structures during oxide growth will better enable scientists to identify defects of certain popular oxide combinations and could help narrow selections of oxides to spur new or more efficient commercial applications.
This research is supported by the U.S. Department of Energy, Office of Science.
The Center for Nanophase Materials Sciences at ORNL is one of the five DOE Nanoscale Science Research Centers supported by the DOE Office of Science, premier national user facilities for interdisciplinary research at the nanoscale. Together the NSRCs comprise a suite of complementary facilities that provide researchers with state-of-the-art capabilities to fabricate, process, characterize and model nanoscale materials, and constitute the largest infrastructure investment of the National Nanotechnology Initiative. The NSRCs are located at DOE's Argonne, Brookhaven, Lawrence Berkeley, Oak Ridge and Sandia and Los Alamos national laboratories. For more information about the DOE NSRCs, please visit nano.energy.gov.
ORNL is managed by UT-Battelle for the Department of Energy's Office of Science.
Contact: Katie Freeman freemanke@ornl.gov 865-574-4160 DOE/Oak Ridge National Laboratory