UC Riverside chemists study quadrupedal molecular machines to provide an answer.
RIVERSIDE, Calif. – Molecular machines can be found everywhere in nature, for example, transporting proteins through cells and aiding metabolism. To develop artificial molecular machines, scientists need to understand the rules that govern mechanics at the molecular or nanometer scale (a nanometer is a billionth of a meter).
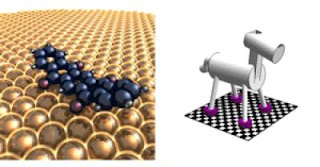
To address this challenge, a research team at the University of California, Riverside studied a class of molecular machines that 'walk' across a flat metal surface. They considered both bipedal machines that walk on two 'legs' and quadrupedal ones that walk on four.
Bartels and colleagues varied the temperature in their experiments to provide the molecular machines with different levels of energy, and studied how the speed of the machines varied as a consequence. They found that a machine with two legs can use tunneling to zip through the surface corrugation. But a machine with four (or potentially more) legs is not able to employ tunneling; while such a machine can coordinate the movement of its hooves in pacing, it cannot coordinate their tunneling, the researchers found.
"Thus, even at the tiniest scale, if you want to transport cargo fast, you need a light and nimble bipedal vehicle," Bartels said. "Larger vehicles may be able to carry more cargo, but because they cannot use tunneling effectively, they end up having to move slowly. Is this discouraging? Not really, because molecular machinery as a concept is still in its infancy. Indeed, there is an advantage to having a molecule move slowly because it allows us to observe its movements more closely and learn how to control them."
Study results appeared online last week in the Journal of the American Chemical Society, and will appear in print in an upcoming issue of the journal.
Next, the researchers plan to develop molecular machines whose motion can be controlled by light.
Currently, molecular machines are being studied intensely for their functions in biology and for their therapeutic value. For example, patients with GERD (Gastroesophageal reflux disease) are prescribed proton pump inhibitors, which slow the pumping action of biological molecular machines, thus reducing stomach acid levels.
"Generally, scientists' picture of the working of such biological molecular machinery completely disregards tunneling," Bartels said. "Our study corrects this perception, which may, in turn, lead to novel ways of controlling or correcting the behavior of biological molecular machines."
Artificial molecular machines are of interest to the microelectronic industry in its quest for smaller and smaller active elements in computers and for data storage. Artificial molecular machines potentially can also operate inside cells like their biological counterparts, greatly benefiting medicine.
Bartels's lab used the following molecules in the study: anthraquinone and pentaquinone (both bipedal); and pentacenetetrone and dimethyl pentacenetetrone (both quadrupedal). ###
The research was made possible by dedicated instrumentation developed and built in the Bartels lab. Bartels specializes in developing scanning tunneling microscopy instrumentation and applying it to molecular systems. Besides the Department of Chemistry, he holds appointments in the departments of physics, electrical engineering, mechanical engineering and the program in materials science and engineering.
He was joined in the study by the following researchers at UCR: postdoctoral scholar Zhihai Cheng; undergraduate student Eric S. Chu; graduate students Dezheng Sun, Daeho Kim, Yeming Zhu, MiaoMiao Luo, Greg Pawin, Kin L. Wong, Ki-Young Kwon and Robert Carp; and Michael Marsella, an associate professor of chemistry. Carp, who works in Marsella's lab, made dimethyl pentacenetetrone; the other chemicals used in the study are commercially available.
The research was supported by a Department of Energy grant to Bartels and a National Science Foundation (NSF) grant to Bartels and Marsella. The latter grant was rated in a recent review of the NSF Division of Chemistry as "an exemplar of excellence in support of the Division's investment in research, education, and infrastructure."
The University of California, Riverside (www.ucr.edu) is a doctoral research university, a living laboratory for groundbreaking exploration of issues critical to Inland Southern California, the state and communities around the world. Reflecting California's diverse culture, UCR's enrollment of about 18,000 is expected to grow to 21,000 students by 2020. The campus is planning a medical school and has reached the heart of the Coachella Valley by way of the UCR Palm Desert Graduate Center. The campus has an annual statewide economic impact of more than $1 billion.
A broadcast studio with fiber cable to the AT&T Hollywood hub is available for live or taped interviews. To learn more, call (951) UCR-NEWS.
Contact: Iqbal Pittalwala iqbal@ucr.edu 951-827-6050 University of California - Riverside
















No comments:
Post a Comment