Researchers from the Eindhoven University of Technology and the University of Ulm have made the first high-resolution 3D images of the inside of a polymer solar cell. This gives them important new insights in the nanoscale structure of polymer solar cells and its effect on the performance. The findings were published online in Nature Materials on Sunday 13 September.
The investigations shed new light on the operational principles of polymer solar cells.
Cost-effective, flexible and lightweight
Nanoscale mixing
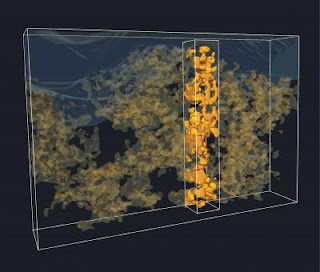
The importance of the degree of mixing was clearly demonstrated by visualization of the structure of these blends in three dimensions. Traditionally such visualization has been extremely challenging, but by using 3D electron tomography, the team has been able to resolve the mixing with unprecedented detail on a nanoscale. From these images the researchers at the Institute of Stochastics in Ulm have been able to extract typical distances between the two components, relating to the efficiency of charge generation, and analyze the percolation pathways, that is, how much of each component is connected to the electrode. These quantitative analyses of the structure matched perfectly with the observed performance of the solar cells in sunlight.
Future
Even though these hybrid polymer solar cells are among the most efficient reported to date for this class, their power conversion efficiency of 2% in sunlight must be enhanced to make them really useful. This will be realized by improving the control over the morphology of the photoactive blend, for example by creating polymers that can interact with the metal oxide and by developing polymers or molecules that absorb a larger part of the solar spectrum. At such point, the intrinsic advantages of hybrid polymer solar cells in terms of low cost and thermal stability of the nanoscale structure could be fully exploited. ###
Publication
The publication "The effect of three-dimensional morphology on the efficiency of hybrid polymer solar cells", by Stefan Oosterhout et al. can be found at DOI 10.1038/NMAT2533.
The research was conducted at the Eindhoven University of Technology and the University of Ulm. It was funded by the Joint Solar Programme of FOM, NWO, and the Shell Research Foundation, the Deutsche Forschungsgemeinschaft, SenterNovem, and the Dutch Polymer Institute.
Contact: René Janssen r.a.j.janssen@tue.nl 31-622-455-438 Eindhoven University of Technology















No comments:
Post a Comment