Gold and sulfur atoms tend to aggregate in specific numbers and highly symmetrical geometries. Sometimes these clusters are called "superatoms" because they can mimic the chemistry of single atoms of a completely different element.
"This discovery contradicted what most chemists believed was going on – which was that the sulfur atom merely sat atop the uppermost gold layer, bound to three adjacent metal atoms," said Whetten.
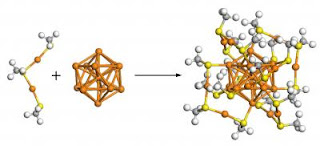
The researchers found that the 102-atom gold cluster was a "superatom" with a core of 79 gold atoms arranged into a truncated decahedron: two pyramids with pentagonal bases joined together into a faceted shape, but with the pyramids' tips chopped off. Around the core, 23 gold atoms formed an unusual pattern, joining the thiolates in shapes that resemble handles.
The results confirmed the "divide and protect" structure first predicted by team member Hannu Häkkinen, a professor at the University of Jyväskylä and former senior research scientist at Georgia Tech in the laboratory of Uzi Landman. Häkkinen and Henrik Grönbeck of the Chalmers University of Technology previously proposed that a cluster of 38-atom gold contained a central metallic core of 14 gold atoms and a protective layer of 24 gold atoms bound to sulfur.
"In 2006, we predicted that gold atoms in this bonding motif were divided in two groups – those that made the metal core and those that helped to protected it," explained Häkkinen. "Now there was evidence that this was true."
In the study reported in PNAS, the researchers found that the clusters were stable because the surface gold atoms in the core each had at least one surface-chemical bond and the gold core exhibited a strong electron shell closing.
With the 102-atom gold cluster, each gold atom in the cluster donated one valence electron. Forty-four of those electrons were immobilized in bonds between gold atoms and thiolates, leaving 58 electrons to fill a shell around the "superatom." In this configuration, the cluster wouldn't benefit from adding or shedding electrons, which would destabilize its structure. This process is similar to what happens in noble gases, which are chemically inert because they have just the right number of electrons to fill a shell around each atom's nucleus.
Associated with the filled electron shell, the gold-thiolate compound also had a major energy gap to unoccupied states. The calculated energy gap between the highest occupied molecular orbital and the lowest unoccupied molecular orbital states for the 102-atom compound was significant – 0.5 electron volts. Metals typically have a gap of zero, so this gap indicates an atypical electronic stability of the compound, explained Whetten.
Besides the 102-atom compound, the researchers also determined the electronic structures for 11-, 13- and 39-atom gold cluster compounds. They found that the 11- and 13-gold atom clusters form closed electronic shells with 8 electrons and the 39-atom gold clusters with 34.
"The theoretical concepts published in this paper provide a solid background for further understanding of the distinct electrical, optical and chemical properties of the stable mono-layer-protected gold nanoclusters," said Whetten, whose funding for this research came from the National Science Foundation and the U.S. Department of Energy. Former Georgia Tech graduate student Ryan Price and current graduate student James Bradshaw also contributed to this work.
The study also shows that experimentally well-characterized, structure-resolved, thermodynamically stable species of thiolate-, phosphine-halide-, and phosphine-thiolate-protected gold nanoparticles share common factors underlying their stability.
Once this initial work was completed, the researchers started predicting the structures of other stable gold cluster compositions that are still awaiting a precise structure determination.
In the March 26 issue of the Journal of the American Chemical Society, the research team predicted the structure for a cluster containing 25 gold atoms. They determined that the structure was comprised of an icosahedron-like 13-atom gold core protected by six "V-shaped" long units, creating a "divide and protect" composition. The structural prediction was recently confirmed by another group's experimental work.
"We now have a unified model that provides a solid background for nanoengineering ligand-protected gold clusters for applications in catalysis, sensing, photonics, bio-labeling and molecular electronics," said Häkkinen. ###
Additional authors on the PNAS paper included Michael Walter, Jaakko Akola and Olga Lopez-Acevedo of the University of Jyväskylä; and Pablo Jadzinsky, Guillermo Calero and Christopher Ackerson of Stanford University.
Contact: Abby Vogel avogel@gatech.edu 404-385-3364 Georgia Institute of Technology Research News
Tags: Nano or Nanotechnology and Nanotech
















No comments:
Post a Comment