CHAMPAIGN, Ill. — The inventors of self-healing plastic have come up with another invention: a new way of doing chemistry.
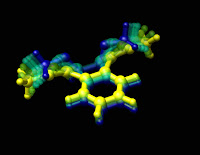 | An overlay of images at successive stages of force-induced chemical change. The blue image is the start of the reaction. The yellow image represents the end of the reaction. Graphic by Ashley Levato. |
Potential applications include materials that more readily repair themselves, or clearly indicate when they have been damaged.
“This is a fundamentally new way of doing chemistry,” said Jeffrey Moore, a William H. and Janet Lycan Professor of Chemistry at Illinois and corresponding author of a paper that describes the technique in the March 22 issue of the journal Nature.
“By harnessing mechanical energy, we can go into molecules and pull on specific bonds to drive desired reactions,” said Moore, who also is a researcher at the Frederick Seitz Materials Laboratory on campus and at the university’s Beckman Institute for Advanced Science and Technology.
The directionally specific nature of mechanical force makes this approach to reaction control fundamentally different from the usual chemical and physical constraints
To demonstrate the technique, Moore and colleagues placed a mechanically active molecule – called a mechanophore – at the center of a long polymer chain. The polymer chain was then stretched in opposite directions by a flow field created by the collapse of cavitating bubbles produced by ultrasound, subjecting the mechanophore to a mechanical tug of war.
“We created a situation where a chemical reaction could go down one of two pathways,” Moore said. “By applying force to the mechanophore, we could bias which of those pathways the reaction chose to follow.”
One potential application of the technique is as a trigger to divert mechanical energy stored in stressed polymers into chemical pathways such as self-healing reactions.
In the original self-healing concept, microcapsules of healing agent are ruptured when a crack forms in the material. Capillary action then transports the healing agent to the crack, where it mixes with a chemical catalyst, and polymerization takes place.
With new mechanical triggers, however, mechanical energy would initiate the polymerization directly, thereby skipping many steps. The cross-linking of neighboring chains would prevent further propagation of a crack and avoid additional damage.
“We have demonstrated that it is now possible to use mechanical force to steer chemical reactions along pathways that are unattainable by conventional means,” Moore said. “We look forward to developing additional mechanophores whose chemical reactivity will be activated by external force.”
The other authors of the paper besides Moore are graduate student and lead author Charles Hickenboth, aerospace engineering professor Scott White, materials science and engineering professor Nancy Sottos, and research chemists Scott Wilson and Jerome Baudry. White, Sottos and Moore co-invented self-healing plastic.
The work was supported by the U.S. Air Force Office of Scientific Research and the Petroleum Research Fund.
Editor’s note: To reach Jeffrey Moore, call 217-244-4024; e-mail: jsmoore@uiuc.edu.
James E. Kloeppel, Physical Sciences Editor 217-244-1073 kloeppel@uiuc.edu
News Bureau, University of Illinois at Urbana-Champaign 807 South Wright Street, Suite 520 East, Champaign, Illinois 61820-6261 Telephone 217-333-1085, Fax 217-244-0161, E-mail news@uiuc.edu
Technorati Tags: nanofibers or Nanoscientists and Nano or Nanotechnology and nanoparticles or Nanotech and nanotubes or nanochemistry and nanoscale or nanowires and Nanocantilevers or nanometrology and University of Illinois at Urbana-Champaign or mechanical force and chemical reactions or polymer chain

















No comments:
Post a Comment