Theo Odijk, you win. The professor of biotechnology at Delft University of Technology in the Netherlands has a new best friend in Rice University's Matteo Pasquali.
Together with collaborators at the French National Center for Scientific Research (CNRS), the University of Bordeaux, France, and Vrije University, Amsterdam, the Rice professor and his team have settled a long-standing controversy in the field of polymer dynamics: The researchers proved once and for all that Odijk was correct in proclaiming that a little flexibility goes a long way for stiff filaments in a solution.
The study in the current issue of the journal Science shows that even a small ability to bend gives nanotubes and other tiny, stiff filaments the means to navigate through crowded environments, or even such fixed networks as cell matrices.
The work by Pasquali, a professor in chemical and biomolecular engineering and in chemistry, may bring about new ways to influence the motion of tiny filaments by tailoring their stiffness for a given environment.
Nanotubes are being studied for potential use in all kinds of sensing, even in the seemingly disparate fields of biological applications and oil exploration. In both, the ability of nanotubes and other fine, filamentous particles to move through their environments is critical, Pasquali said.

Matteo Pasquali | Understanding the motion of a single, flexible polymer chain in a network has been key to scientific advances by Odijk and others on, for example, the behavior of DNA. The Rice researchers expect their revelation to have no less impact.
Pasquali and lead author Nikta Fakhri, a former graduate student at Rice now doing postdoctoral research at the University of Gottingen, Germany, set out to break the deadlocked theories by Odijk and two other scientists who disagreed on the Brownian motion of stiff filaments in a crowded environment, and whether stiffness itself played any part.
"There's a long-standing, fundamental question: How does this threadlike object move when it gets crowded? It could be crowded because it's in a gel, or because there are a lot of threadlike objects with it -- which to that one object looks like a gel," he said. |
Crowding constrains the ability of a filament to travel. Think of trying to get from the back to the front of a crowded bus; it takes a certain amount of agility to weave your way through the packed bodies. "It turns out that with a little flexibility, a filament can explore the space around it much more effectively," Pasquali said.
That becomes important when the goal is to get filaments to find and enter a cellular pore to deliver a dose of medication or to act as a fluorescent sensor.
"If you look at the human body, they say we're made of 60 percent water, but we don't slosh around," Pasquali explained. "That's because the water is trapped in pores. Almost all the water in our body is in gel-like structures: inside our cells, which are laden with filamentous networks, or in the interstitial fluid surrounding these cells. We are a big, squishy, porous medium. We need to understand how the nanoparticles move in this medium."
Pasquali and Fakhri mimicked biological networks by using varying concentrations of agarose gel, a porous material often used as a filter in biochemistry and molecular biology for DNA and proteins. The gel forms a matrix of controllable size through which molecules can move.
Nanotubes served as a stand-in for any type of filament, albeit one whose stiffness can be controlled. Like a PVC pipe in the macro world, nanotubes get stiffer as they get thicker; but even the stiffest tubes can flex a bit with length, and these tubes were thousands of times longer than they were wide.
The study started somewhat serendipitously when co-author Laurent Cognet, a researcher at CNRS and the University of Bordeaux, tried to immobilize nanotubes in agarose gels. He noticed in a failed experiment that the nanotubes moved in a "funny way" and discussed it with Pasquali.
Pasquali asked whether the nanotubes were reptating -- scientist lingo for a snakelike motion -- and Cognet said yes. Fakhri, who was studying the dynamics of nanotubes, traveled to the Bordeaux laboratory of Cognet and co-author Brahim Lounis to capture images of the nanotubes in motion.
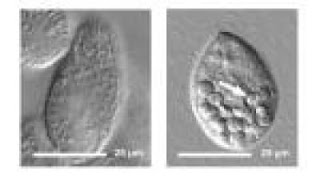
The resulting spectroscopic and direct still and video images of 35 fluorescent single-walled nanotubes showed them snaking through the gel, probing pores and paths. The nanotubes, like all filaments, obeyed the rules of thermal-induced Brownian motion; they were pushed and pulled by the ever-changing states of the molecules around them.
The research established that flexibility significantly enhances the nanotubes' ability to navigate around obstacles and speeds up their exploration.
Pasquali said Fakhri doggedly pursued her analysis of the nanotubes' motion through computerized image recognition and motion tracking, as well as old-fashioned pencil-and-paper dynamical analysis. He said his longtime collaborator, co-author Frederick MacKintosh, a theoretical physicist at Vrije University, was a tremendous help. MacKintosh has been studying the dynamics of biological networks for nearly two decades.
Pasquali intends to replace the gel with real rocks to see how nanotubes, which can be used as oil-detecting sensors, move in a more structured environment. "Rocks can be a little more complicated," he said. "The question here is, what can nanotubes do better than nanoparticles? The answer may be that slender nanotubes may interact with electromagnetic fields more strongly than other nanoparticles of the same volume."
The National Science Foundation Center for Biological and Environmental Nanotechnology, the Welch Foundation, the Advanced Energy Consortium, the Région Aquitaine, the Agence National pour la Recherche, the European Research Council and the Dutch Foundation for Fundamental Research on Matter supported the work.
Contact: David Ruth
druth@rice.edu 713-348-6327
Rice University