Powerful new microscopes able to resolve DNA molecules with visible light, superfast computers that use light rather than electronic signals to process information, and Harry Potteresque invisibility cloaks are just some of the many thrilling promises of transformation optics. In this burgeoning field of science, light waves can be controlled at all lengths of scale through the unique structuring of metamaterials, composites typically made from metals and dielectrics - insulators that become polarized in the presence of an electromagnetic field. The idea is to transform the physical space through which light travels, sometimes referred to as "optical space," in a manner similar to the way in which outer space is transformed by the presence of a massive object under Einstein's relativity theory.
So far transformation optics have delivered only hints as to what the future might hold, with a major roadblock being how difficult it is to modify the physical properties of metamaterials at the nano or subwavelength scale, mainly because of the metals. Now, a team of researchers with the U.S. Department of Energy (DOE)'s Lawrence Berkeley National Laboratory (Berkeley Lab) and the University of California (UC) Berkeley have shown it might be possible to go around that metal roadblock.
Says Liu, who was the lead author of the paper and is a post-doctoral researcher in Zhang's UC Berkeley group, "In addition to the 180 degree plasmonic bend and the plasmonic Luneburg lens, our approach should also enable the design and production of beam splitters and shifters, and directional light emitters. The technique should also be applicable to the construction of integrated, compact optical data-processing chips."
Zhang and his research group have been at the forefront of transformation optics research since 2008 when they became the first group to fashion metamaterials that were able to bend light backwards, a property known as "negative refraction," which is unprecedented in nature. In 2009, he and his group created a "carpet cloak" from nanostructured silicon that concealed the presence of objects placed under it from optical detection.
For this latest work, Zhang and Liu with Zentgraf and Bartal departed from the traditional transformation optics focus on propagation waves and instead focused on the SPPs carried in near-field (subwavelength) region.
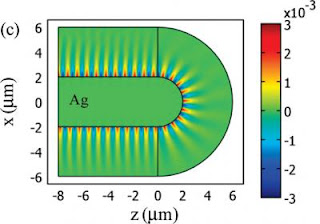
"The intensity of SPPs is maximal at the interface between a metal and a dielectric medium and exponentially decays away from the interface," says Zhang. "Since a significant portion of SPP energy is carried in the evanescent field outside the metal, that is, in the adjacent dielectric medium, we proposed to control SPPs by keeping the metal property fixed and only modifying the dielectric material based on the transformation optics technique."
Full-wave simulations of different transformed designs proved the proposed methodology by Zhang and his colleagues correct. It was furthermore demonstrated that if a prudent transformational plasmon optics scheme is taken the transformed dielectric materials can be isotropic and nonmagnetic, which further boosts the practicality of this approach. The demonstration of a 180 degree bend plasmonic bend with almost perfect transmission was especially significant.
"Plasmonic waveguides are one of the most important components/elements in integrated plasmonic devices," says Liu. "However, curvatures often lead to strong radiation loss that reduces the length for transferring an optical signal. Our 180 degree bend plasmonic bend is definitely important and will be useful in the future design of integrated plasmonic devices."
Compared with silicon-based photonic devices the use of plasmonics could help to further scale- down the total size of photonic devices and increase the interaction of light with certain materials, which should improve performance.
"We envision that the unique design flexibility of the transformational plasmon optics approach may open a new door to nano optics and photonic circuit design," Zhang says. ###
This research was supported by the U.S. Army Research Office and the National Science Foundation's Nano-scale Science and Engineering Center.
Berkeley Lab is a U.S. Department of Energy (DOE) national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California for the DOE Office of Science. Visit our Website at: www.lbl.gov.
Contact: Lynn Yarris lcyarris@lbl.gov 510-486-5375 DOE/Lawrence Berkeley National Laboratory