An over-the-counter prostate cancer test kit could be coming to a pharmacy near you, thanks to the collaborative work of a University of Central Florida chemist and M.D. Anderson Cancer Center Orlando researchers.
UCF's Qun "Treen" Huo and M.D. Anderson-Orlando's Dr. Cheryl Baker and Jimmie Colon teamed up about 18 months ago with a very ambitious plan. Huo wanted to develop an effective, inexpensive test to screen for prostate cancer that would be easy enough to use at home or a local pharmacy.
"Now cancer tests are so inconvenient and expensive, and a lot of people don't have insurance, so they are not likely to test if they have no symptoms," Huo said. "Cancer is really scary because there aren't a lot of symptoms in the early stages. So I said, 'Why not create a test that is easy and inexpensive? Then more people can test and catch cancer early so it can be treated early.'"
During a test, if cancer-producing proteins are detected at a significant level, the consumer would be directed to see a doctor.
"Think of it like a pregnancy test," Huo said. "It's the same principle. Women use it to find out if they are pregnant, but once they see the results at home, they go to the doctor to be sure."
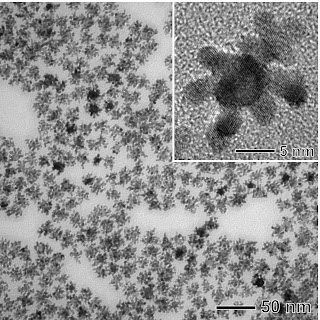
The cancer-related protein marker that the gold nanoparticles seek out in Huo's research is the same one screened for by the FDA-approved Prostate-Specific Antigen (PSA) test. The PSA has a good track record as a protein marker for detecting prostate cancer and has been used by physicians for years. Huo said her new technique is much more sensitive and accurate than the PSA and all current techniques used in diagnostic labs.
"What's different is the technology," Huo said. "It's very simple. The dynamic light-scattering technique is highly sensitive and can pick up even the smallest trace amount of protein markers."
That's why the technique also can help doctors track any resurgence of cancer once a surgery is performed to remove it.
Dr. Baker, director of M.D. Anderson-Orlando's Cancer Research Institute, collaborates with Huo by offering her expertise in cancer research and by providing human blood and serum samples to test Huo's technique.
"The excitement for us here at M. D. Anderson-Orlando is that we can easily test the validity of the technique in our cancer research program and then on our own patients in a clinical trial," Baker said. "We are optimistic that we can begin clinical trials with this test within the next two years."
Huo said that the technique is still years from commercialization, but that in three to five years an over-the-counter test kit for prostate cancer is likely. The technique also can be easily adapted to test for many different types of cancer – Huo plans to focus first on ovarian and breast cancer. ###
Huo, who joined UCF in 2005, is an associate professor at the NanoScience Technology Center and Department of Chemistry. She teaches and conducts research in nanomaterials chemistry and developing applications for nanoparticle materials. Her interest in cancer research stems from watching friends and relatives battle a variety of cancers with little warning because symptoms are difficult to detect. Much of her research is funded through the National Science Foundation.
UCF Stands For Opportunity --The University of Central Florida is a metropolitan research university that ranks as the 5th largest in the nation with more than 50,000 students. UCF's first classes were offered in 1968. The university offers impressive academic and research environments that power the region's economic development. UCF's culture of opportunity is driven by our diversity, Orlando environment, history of entrepreneurship and our youth, relevance and energy. For more information visit news.ucf.edu
M. D. Anderson Cancer Center Orlando, part of Orlando Health, is affiliated with The University of Texas M. D. Anderson Cancer Center in Houston. U.S. News & World Report recently ranked M. D. Anderson Cancer Center as the top cancer treatment center in the U.S. and has ranked it as one of the top two cancer centers for the past 13 years. Orlando Health, a 1,780-bed community-owned, Florida not-for-profit organization established in 1918, annually serves nearly 2 million Central Florida residents and more than 4,500 international patients. More information is available at orlandohealth.com and mdacco.com.
Contact: Zenaida Gonzalez Kotala zkotala@mail.ucf.edu 407-823-6120 University of Central Florida